Glossary
Atmospheric Corrosion
Atmospheric corrosion refers to the corrosive action that occurs on the surface of a metal in an atmospheric environment. It occurs when the surface is wet by moisture formed due to rain, fog and condensation. Atmospheric corrosion is a complex process involving a large number of interacting and constantly varying factors, such as weather conditions, air pollutants, material conditions, etc. The combined effect of these factors results in a great variations in corrosion rates.
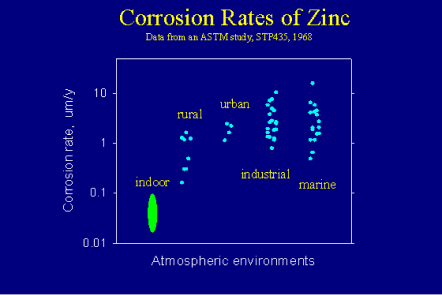
As shown in Figure 3, the corrosion rate of zinc in atmospheric environments may vary from as low as about 0.1 µm/year in indoor environments to as high as over 10 µm/year in some industrial or marine environments, about two orders of magnitude of variation. This means that a G60 galvanized steel, with about 13 µm coating thickness on each side, would have a corrosion life of more than 100 years in the least corrosive environment but only for about one year in an extremely corrosive environment.
Most of the factors influencing the atmospheric corrosion of metals are not controllable and only some of them can be monitored with reasonable accuracy, consistency and cost. In addition, depending on the specific geographic setting and application conditions, the microscopic environment, which actually determines the corrosion rate, may significantly differ from the macroscopic environment characteristic of the geographic location [1]. Furthermore, as weather changes with time from year to year, the corrosion rate also tends to vary. It is thus not possible in general to accurately predict corrosion rates in atmospheric environments.
Coating Life
Coating life is the total time required for the zinc coating to decrease to zero thickness due to corrosion. The model does not consider the variation in the composition and thickness of the Zn-Fe alloys layers which may consist of significant portion of the batched galvanizing coatings. Coating life measured in years, is estimated from the zinc coating thickness and the corrosion rate calculated by the prediction model.
Coating Thickness
Coating Thickness is the thickness of the zinc coating on the steel substrate. Coating thickness can be expressed in terms of weight or actual thickness. The factors for conversion between different units are:
1 oz/ft2 = 305 g/m2 = 30.5 mg/cm2 = 1.70 mil = 43.2 µm
1 mm = 1000 µm = 0.039 in
1 in = 1000 mil = 25.4 mm
Continuous Galvanizing coating thickness categories include:
G40/Z120 = 8.5 µm
G60/Z180 = 12.7 µm
G90/Z275 = 19.4 µm
Corrosion Rate
Corrosion Rate, R, is defined as the amount of corrosion loss in thickness per year: R= d/t
Where d is the total amount of thickness lost due to corrosion in micrometers, t, is the number of years during which the loss occurred. The corrosion rate is most commonly expressed in the units of µm/year. It may also be expressed in terms of weight loss such as mg/dm2*day, g/m2, oz/ft2, etc.
The corrosion rate expressed in thickness loss or weight loss is meaningful for evaluation of the situations where the metal surface corrodes uniformly across the exposed area, i.e. general corrosion form. Large amounts of field exposure results indicate that the corrosion form of zinc or zinc coated steel is this general corrosion or uniform corrosion.
The corrosion rate expressed in the above function is a time-averaged rate over at least one year and may differ from rates calculated over short periods. Short-term corrosion rates in any outdoor atmospheric environment tend to greatly fluctuate as environmental conditions vary from day to day and from season to season. Short term data is thus generally less representative of the typical corrosion rate of the environment.
Corrosivity of Atmosphere
The corrosivity of atmosphere refers to the extent of corrosion suffered by a metal caused by exposing it in the atmosphere. Traditional, corrosivity of atmosphere is categorized according to the geological-social conditions: rural, urban, industrial and marine etc. More recently, it is also classified more quantitatively according to time of wetness and airborne pollutants in terms of sulfur dioxide and salt contents. The corrosivity of an atmosphere toward a particular metal is measured by the corrosion rate, R, of the metal in the atmosphere. According to the classification of ISO [2] the corrosivity of atmospheres to zinc is divided into five categories:
| C1 | R ≤ 0.1 µm/y | R ≤ 0.0023 oz/ft2/y | R ≤ 0.0023 oz/ft2/y |
| C2 | 0.1 < R ≤ 0.7 | 0.0023 < R ≤ 0.016 | 0.71 < R ≤ 4.9 |
| C3 | 0.7 < R ≤ 2.1 | 0.016 < R ≤ 0.049 | 4.9 < R ≤ 15 |
| C4 | 2.1 < R ≤ 4.2 | 0.049 < R ≤ 0.097 | 15 < R ≤ 30 |
| C5 | 4.2 < R ≤ 8.4 | 0.097 < R ≤ 0.19 | 30 < R ≤ 59 |
Category C1 involves generally climate-controlled indoor atmospheres in which the corrosion rate is very low while category C5 includes atmospheric environments which are very corrosive to zinc such as by the sea shore or heavily polluted with SO2 or extremely humid conditions. In the ISO Standard, the corrosivity of atmospheres is correlated to three key factors: the time of wetness, t, the sulfur dioxide content, P, and the air-borne salinity, S. Table 3 shows the ISO classification of corrosivity in terms of zinc corrosion rate in relation to these three factors. In addition to these factors, other atmospheric factors may also play significant roles in determining the corrosion rate of zinc such that the corrosion rate can still vary in a certain range for a given combination of the three specified factors.
Indoor
Indoor is an environment condition where the air is shielded from the outdoor air
Rain or Precipitation
The total amount of rain or precipitation in a year or annual precipitation measured in units of mm or inch.
Relative Humidity
Relative Humidity, RH, is defined as the ratio of the quantity of water vapor present in the atmosphere to the saturation quantity at a given temperature, and it is expressed as %. RH in open atmosphere greatly fluctuates during the day and from day to day due to climatic changes. RH is an important factor which together with temperature variation, causes moisture condensation on metal surface.
Salinity
Airborne salinity refers to the content of gaseous and suspended salt in the atmosphere. It is measured by the concentration in the air in units of µg/m3. Since it is the salt that is deposited on the metal surface that affects the corrosion, it is often measured in terms of deposition rate in units of mg/m2/day. The pollution levels can also be measured in terms of the concentration of the dissolved salt in rain water. Although the surface salt deposition rate and the salt concentration in rain water results from the salt content in the air, the direct correlation between these different quantities has not been found. The ISO classification of salinity levels expressed as annual averages for outdoor atmospheres is given in the following table.
| Category | Deposition rate of NaCL, mg/m2*day | Typical Environment |
|---|---|---|
| S0 | S ≤ 3 | Non-Coastal |
| S1 | 3 < S ≤ 60 | Coastal Environment |
| S2 | 60 < S ≤ 300 | Coastal Environment |
| S3 | 300 < S ≤ 1500 | Within 200m from sea |
Sheltered Conditions
A sheltered condition is referred to the situations where the metal surface is exposed to the outdoor air but is shielded from the rain. In general, sheltering reduces the corrosion rate of zinc coatings. At locations close to the sea water the corrosion rate under a sheltered condition can be higher than that openly exposed because of the increased time of wetness under the sheltered condition.
Sulfur Dioxide
Sulfur Dioxide, SO2, is a gaseous pollutant in the atmosphere resulting predominantly from the burning of coal, oil, and gasoline and has been identified as one of the most important air pollutants which contribute to the corrosion of metals. It is usually measured in terms of its concentration in air in units of µg/m3. Since it is the SO2 deposited on the metal surface which affects the corrosion, it is also often measured in terms of deposition rate on the surface in units of mg/m2/day. The pollution levels can also be measured in terms of the concentration of the dissolved SO4 in rain water. Although qualitatively, high SO2 concentration in the air generally results in a large amount of deposition on an exposed surface and a high concentration of dissolved SO4 in rain water, there is not an exact correlation between these different quantities. According to International Standard ISO 9223 [2], the relationship between surface deposition, Pd, and air concentration, Pc could be roughly expressed as: Pd = 0.8 Pc. The ISO classification of pollution by sulphur dioxide for outdoor atmospheres is given in the following table.
| Category | Deposition rate of SO2, mg/m2*day | Concentration of SO2 in air, µg/m3 | Typical Environment |
|---|---|---|---|
| P0 | Pd ≤ 10 | Pc ≤ 12 | Rural |
| P1 | 10 < Pd ≤ 35 | 12 < Pc ≤ 40 | Urban or Industrial |
| P2 | 35 < Pd ≤ 80 | 40 < Pc ≤ 90 | Urban or Industrial |
| P3 | 80 < Pd ≤ 200 | 90 < Pc ≤ 250 | Heavily Polluted |
The P values are the annual averages calculated from continuous measurements for at least one year. P0 is considered to be the background concentration and P3 is considered to be extreme. In general, high sulfur dioxide deposition rate tends to result in high rate of corrosion. However, for two different geographic locations with similar sulfur dioxide deposition rates corrosion rates may significantly differ because many other factors may also play significant roles.
Temperature
Temperature, T, is defined as the annual mean air temperature at which the material is exposed and is expressed in °C or °F.
Time
Time is defined as the length of time measured in units of years in which the material is exposed in an atmosphere.
Time of Wetness
Time of Wetness, TOW, is an estimated parameter based on the length of time when the relative humidity is greater than 80% at a temperature greater than 0°C. It can be expressed as the hours or days per year or the annual percentage of time. Although TOW is used by many researchers as a parameter to correlate corrosion rates with atmospheric factors and although it is used in the ISO Standard, it is not used in this software as an input parameter. It is generally not as readily available as RH and temperature data because it is a secondary quantity derived from values of RH and temperature.
References
- X.G. Zhang, "Corrosion and Electrochemistry of Zinc", Plenum, New York, 1996.
- International Standard, "Corrosion of Metals and Alloys -- Corrosivity of Atmospheres -- Classification", ISO 9223.